Abstract

Background: The protoporphyrias, comprised of erythropoietic protoporphyria (EPP) and X-linked protoporphyria (XLP), are rare photodermatoses characterized by phototoxic reactions and, in a subset of patients, hepatic failure. In 2019, the U.S. Food and Drug Administration approved afamelanotide (Scenesse®, Clinuvel), an α-melanocyte stimulating hormone analogue, dramatically changing the clinical management of patients with EPP/XLP. However, little is known about post-marketing experience with this medication in the U.S. According to current estimates, only a small fraction of U.S. patients eligible for afamelanotide are receiving this drug. The Massachusetts General Hospital (MGH) Porphyria Center is one of the largest programs in the U.S. that offers afamelanotide.
Methods: This was a single-center retrospective cohort study of all patients with EPP/XLP treated at the MGH Porphyria Center from March 2021 through May 2022. Afamelanotide is administered subcutaneously every two months, with a maximum of 6 implants per year.
Main outcomes: Time until phototoxic reaction and frequency and severity of symptoms pre- versus post-treatment with afamelanotide, changes in quality of life with afamelanotide, and side effects of afamelanotide.
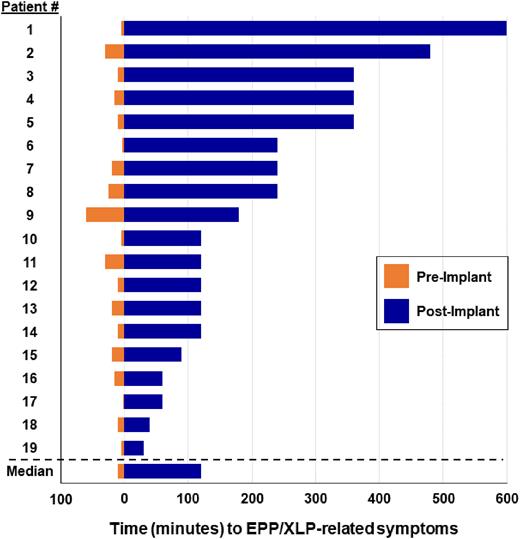
Results: A total of 30 patients with EPP/XLP were evaluated during the period of interest, 24 of whom (n=23 [EPP], n=1 [XLP]) received at least one afamelanotide implant. The median age for patients who received afamelanotide was 40 years (interquartile range [IQR], 32-57), and 19 (79%) were female. The median number of implants received was 4 (IQR, 2-5). Among the 19 patients who received at least 2 implants, the median time to symptom onset after light exposure was 10 minutes (IQR, 5-20) prior to initiation of afamelanotide, and 120 minutes (IQR, 105-300) after initiation of afamelanotide (P<0.001). All patients who received at least 2 doses of afamelanotide reported a decrease in frequency and severity of phototoxic reactions. Among patients who had received afamelanotide within the last two months, 84% reported that their quality of life had improved either "very much" or "a lot."
Adverse events were generally mild and included nausea, vomiting, flushing, and bruising at the implant site. Of the 6 patients who did not receive afamelanotide, one was scheduled to receive afamelanotide after the data lock period, one was receiving afamelanotide at another center, three were on a clinical trial with an oral α-MESH analogue, and one was unable to afford the medication.
Conclusion: In this U.S. single-center cohort, afamelanotide led to a significant increase in median time to phototoxic reaction in patients with EPP/XLP, decrease in symptom frequency and severity, and improvement in quality of life. Afamelanotide was generally well-tolerated with limited side effects.
Disclosures
Leaf:Mitsubishi Tanabe: Other: Advisory Board member; Recordati: Other: Advisory Board member. Hodges:Alnylam: Other: Advisory Board member. Anderson:Alnylam: Consultancy, Other: Advisory Board member, Research Funding; Mitsubishi Tanabe: Consultancy, Other: Advisory Board member, Research Funding; Recordati: Consultancy, Other: Advisory Board member, Research Funding. Dickey:Disc Medicine: Research Funding; Mitsubishi Tanabe: Consultancy; Recordati: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal